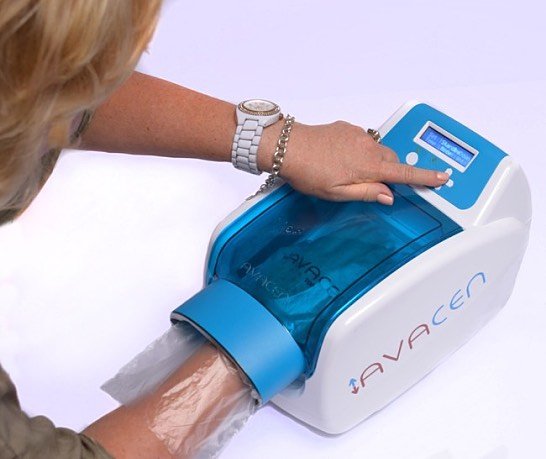
Avacen Medical says its Avacen 100 Class-II medical pain treatment product has received a medical device license from Health Canada to treat fibromyalgia. The San Diego-based biotech firm’s marquee product uses a non-invasive technique for safely infusing heat into the body’s circulatory system.
Avacen says its device relaxes muscles while increasing microcirculation throughout the body, and that unlike other local pain relief medical devices, the Avacen 100’s proprietary action mechanism provides widespread, systemic pain relief.
CEO Thomas Muehlbauer described the device in a press release as “the only OTC [over-the-counter] medical device on the market today able to provide non-invasive, rapid whole body treatment, using a single point of contact. It is the ideal drug-free and safe alternative for relief of muscle and joint pain.”
The device is not for sale in the United States, Canada, Europe or India for the treatment of any non-cleared or non-approved indication.
Canadian federal and provincial healthcare officials are worried about the growing number of opioid drug-related deaths, partly from street fentanyl but also from the abuse of legal prescription painkillers.
“The most exciting aspect of this approval is that the Canadian market has shown high demand and acceptance for innovative new alternatives to pharmaceuticals for treating pain.” said Muehlbauer, whose company already has approval in the European Union to market the Avacen 100 throughout the EU’s 28 member countries. Many of them have rejected prescription drugs — including some available in the United States — for treating fibromyalgia pain.
Besides fibromyalgia’s devastating impact on quality of life, it imposes a huge economic burden on both healthcare providers and people battling the disease.
Avacen cited a recent Canadian study published in the journal BMC Musculoskeletal Disorders showing that fibromyalgia costs patients an average C$3,804 per year. The study, “Fibromyalgia-related costs and loss of productivity: a substantial societal burden,” found that besides prescribed medications, two-thirds of study participants also purchased over-the-counter pain medication, and more than half bought natural health products — both included in out-of-pocket costs.
The Avacen 100 device sells for US$2,595, with an estimated operating lifetime of five to 10 years. It features a patient compliance enhancing game mode, a custom hand sensor, adjustable heat and time controls, an easy-to-read LCD screen and device usage monitor.
The procedure is quite simple; users put their hand inside the Avacen 100 vacuum chamber and rest it on a heated pad for 10 to 30 minutes. Avacen says more than 300,000 treatments have been completed without adverse events.