A new clinical trial of a non-drug approach for treating fibromyalgia is being conducted by researchers at the University of Cincinnati (UC). The study will investigate the use of a proprietary electrode-emitting headband device called Cefaly, currently in use for treating migraine headaches, to assess its potential therapeutic effect on people with the chronic and painful fibromyalgia.
In addition to pain, fibromyalgia symptoms typically include fatigue, sleep disturbance, and cognitive thinking. Fibromyalgia afflicts approximately 2 percent of the U.S. population, according to the U.S. Centers for Disease Control and Prevention, and is about seven times more common in women than in men.
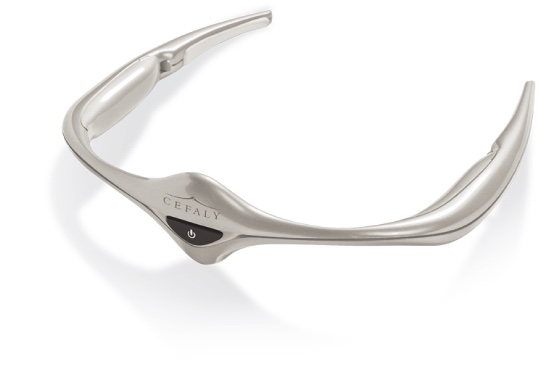
The Cefaly headband is FDA-approved for treatment of migraine headaches. In its standard use, Cefaly is positioned on the forehead using an adhesive electrode. Precise impulses are produced which act on the trigeminal nerve to prevent migraine attacks. UC is the first research center in America to test the Cefaly device in a trial for patients with fibromyalgia.
Image courtesy Cefaly
The device delivers electrodes of neuro-stimulation to limit the pain signals from the nerve center in the brain. In migraine sufferers, this may help reduce the frequency and severity of headaches, providing a non-drug alternative treatment for painful and often debilitating migraine attacks.
 Lesley Arnold, M.D., a UC Health physician and professor of psychiatry and behavioral neuroscience at the University of Cincinnati, has conducted several studies investigating both medication and non-drug treatments for fibromyalgia, and says the current trial will use the Cephaly headband to assess whether the electrode therapy can have a similar effect on fibromyalgia symptoms as it does on migraines.
Lesley Arnold, M.D., a UC Health physician and professor of psychiatry and behavioral neuroscience at the University of Cincinnati, has conducted several studies investigating both medication and non-drug treatments for fibromyalgia, and says the current trial will use the Cephaly headband to assess whether the electrode therapy can have a similar effect on fibromyalgia symptoms as it does on migraines.
“People with fibromyalgia can experience widespread musculoskeletal pain accompanied by fatigue, sleep disturbances, as well as memory issues, anxiety, and depression,” said Arnold, who is also director of the UC Women’s Health Research Program and Fibromyalgia Treatment Program. “Research has shown that fibromyalgia amplifies painful sensations by affecting the way your brain processes pain signals. Symptoms sometimes begin after significant psychological stress or physical trauma, but can also gradually accumulate over time with no single triggering event.”
The trial is open to adults between the ages of 18 and 65 who have been diagnosed with fibromyalgia. All enrolled participants will receive the headband devices provided by Cefaly, which is sponsoring the trial.
“Since this is a pilot study, there is no placebo,” said Arnold, who cites no conflict of interest. “Everyone’s going to get the treatment, and if people report some improvement, then they will go on to do the controlled studies, to try to get approval for it. It’s really a first step.”
Photo courtesy University of Cincinnati
Arnold has already started using the device with patients in her clinic, but the trial will take up to 50 volunteers and will run through the remainder of 2016.
For more information on the trial, contact Kerri Earles at 513-558-7104 or via email at [email protected].