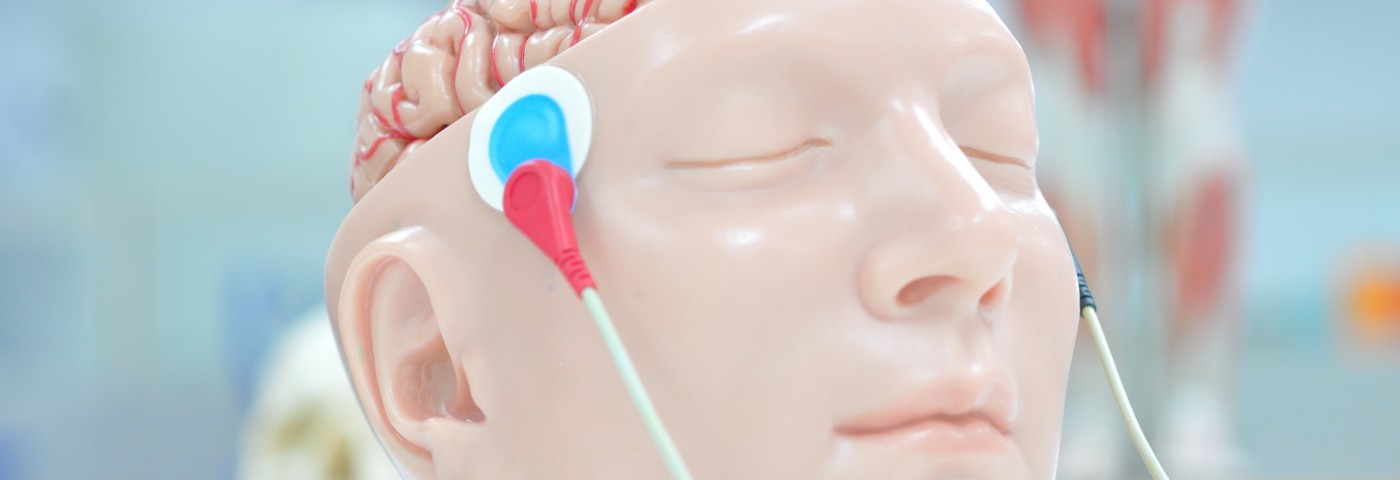
Soterix Medical, Inc., recently announced its PainX platform for the treatment of fibromyalgia and migraine pain, based on its proprietary transcranial Direct Current Stimulation (tDCS) technology, is now available in Europe. The platform recently received CE Mark approval, meaning that it complies with European safety, health, and environmental regulatory requirements.
Invented and developed by a team of doctors and engineers at Soterix Medical, PainX is a non-invasive medical device that stimulates the brain via an external conductive patch. The device delivers a low intensity current to a specified area of the cerebral cortex to control pain perception or to modulate patients’ response to painful experiences.
The device offers a promising treatment alternative for patients suffering from fibromyalgia or migraine, and one without the potential side effects of medications. Residents in the European Union may obtain the system with a physician’s prescription; the therapy is not yet available in the United States or Canada.
According to the company, PainX is the first tDCS system to incorporate adequate safety and control features for clinical use. The device is powered by a battery that connects to a headgear, guiding a current to specific brain areas during an outpatient treatment session.
“We are glad Soterix Medical can bring light into the lives of patients suffering from chronic pain disorders,” Shani San Solo, vice president, Europe Business for Soterix Medical, said in a press release. “The CE approval allows our unique tDCS technology to provide an effective, safe and well-tolerated solution for the severe conditions like fibromyalgia, migraine, and other forms of chronic pain. This approval, coming soon after the CE approval of Soterix Medical 1×1 tDCS for Depression, reinforces the standing of Soterix Medical as the leader in non-invasive neuromodulation.”
Added Dr. Abhishek Datta, CTO of Soterix Medical: “Many chronic pain disorders are associated with neuropathic pain, meaning pain that originates through maladaptive plasticity in the brain. The PainXTM tDCS therapy enhances adaptive plasticity encouraging correction of brain activity. Critical to the validation of a safe and effective treatment for pain, was over 5 years of design and testing of the PainXTM stimulator and headgear.”
The CE mark was awarded based on positive data collected from 14 trials over eight years for chronic pain conditions that included fibromyalgia, migraine, and spinal cord injury. In the majority of the cases, patients experienced quick relief. Results derived from some of these studies also showed that patients experienced a sustained benefit of three to 12 weeks after stimulation with the device. The most common reported side effects included itching and tingling; on rare occasions, patients also reported headaches.