Tuscaloosa, Alabama-based Innovative Med Concepts, a biotech company with a pipeline of novel treatments for fibromyalgia and other related conditions that appear to share a common underlying pathophysiology, has announced it has been granted U.S. Food and Drug Administration Fast Track Designation for the development of IMC-1, the lead drug candidate in its pipeline for treating fibromyalgia.
IMC-1 previously completed a randomized, double-blind, placebo controlled Phase 2 study (PRID-201), showing to have promise for treating pain and other symptoms of fibromyalgia as well as irritable bowel syndrome (IBS), myalgic encephalomyelitis or chronic fatigue syndrome (ME/CFS), chronic neck and back pain, cognitive impairment, and a broad range of other conditions that show strong evidence of sharing the same underlying pathophysiology.
IMC-1 has achieved a milestone with completion of the Fibromyalgia Anti-viral Suppression Trial (the FAST study): a double-blind, placebo-controlled Phase 2 proof-of-concept trial designed to evaluate the hypothesis that tissue-resident herpes virus may be causally related to fibromyalgia symptoms or recurrence. A group of 143 women from 12 U.S. clinics were diagnosed with primary fibromyalgia as defined by the 2010 College of Rheumatology Preliminary Diagnostic Criteria for Fibromyalgia. They were evaluated at various intervals for 16 weeks. IMC is currently working with the FDA to meet all the necessary requirements to start the IMC-1 Phase 3 trials for management of fibromyalgia.
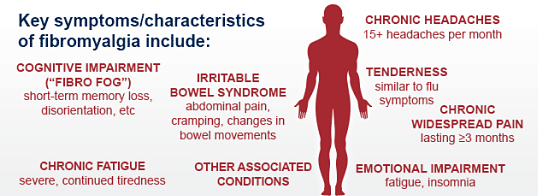
Fibromyalgia is a common and often debilitating musculoskeletal condition that causes chronic widespread pain and fatigue. It is often accompanied by a consistent “cluster” of symptomatic concomitant conditions (such as ME/CFS, joint stiffness, and IBS) that suggest these syndromes share an underlying pathophysiology. Fibromyalgia typically manifests with widespread chronic pain along with a wide range of other symptoms that may include fatigue, headaches, sleep problems, mood changes and an inability to concentrate.
According to the National Fibromyalgia Association, an estimated 3 to 6 percent of people worldwide suffer from the condition — about 10 million in the United States and 220 million people worldwide. However, estimates vary, and IMC notes that it has been suggested fibromyalgia is present in as high as 8 percent of the U.S. population, or 12 million people.
Whatever the actual numbers, despite increased research since the late 1970s, there are no specific lab tests to diagnose fibromyalgia and no cure, and this disabling disease has yet to be fully accepted by both the medical and lay communities. For the most part, the exact cause of heightened pain sensitivity in fibromyalgia patients is poorly understood, although IMC observes that scientists and clinicians can agree that fibromyalgia patients have a problem with central pain processing, and it is generally believed that the central sensitization is secondary to some combination of genetic and environmental factors that predisposes the patient to the disorder. Physical trauma, infection, emotional distress, endocrine disorders and immune activation have also all been hypothesized as potential triggering phenomena in susceptible persons.
IMC observes that modern fibromyalgia therapies have largely focused on altering pain perception, or treating the numerous symptoms of the array of associated conditions, and while some progress has been achieved, there still is much room for improvement. IMC affirms its commitment to developing treatments for fibromyalgia and hopes to one day market a treatment that targets the root cause of the condition.
In the successful Phase II trial in fibromyalgia, IMC-1 was studied to confirm the hypothesis that chronic tissue-resident herpes simplex virus type 1 (HSV-1) may be one of the underlying causes of fibromyalgia and causally related to fibromyalgia symptoms or recurrence. IMC-1 is a novel fixed-dose oral combination anti-viral medication designed to suppress this suspected virus and thus alleviate fibromyalgia symptoms.
IMC says its innovative treatments have emerged from breakthrough scientific discoveries in HSV-1 reaction, the factor leading to many of the symptoms associated with fibromyalgia and its related conditions.
 “We are pleased that the FDA has granted Fast Track status to IMC-1, as it demonstrates their recognition of this condition as a significant unmet medical need,”says William Pridgen, M.D., founder and CEO of Innovative Med Concepts in a release. “IMC-1 has a novel mechanism of action designed to suppress chronic tissue-resident herpes virus, which may have a role in triggering or maintaining symptoms of fibromyalgia. IMC-1 is a fixed-dose combination using proprietary doses of famciclovir, an anti-viral nucleoside analog, and celecoxib, a COX-2 inhibitor that also possesses unique anti-viral activity. Suppressing latent herpes viruses may significantly improve fibromyalgia-related symptoms. We are currently meeting the development requirements to begin Phase 3 trials with IMC-1 in 2017.”
“We are pleased that the FDA has granted Fast Track status to IMC-1, as it demonstrates their recognition of this condition as a significant unmet medical need,”says William Pridgen, M.D., founder and CEO of Innovative Med Concepts in a release. “IMC-1 has a novel mechanism of action designed to suppress chronic tissue-resident herpes virus, which may have a role in triggering or maintaining symptoms of fibromyalgia. IMC-1 is a fixed-dose combination using proprietary doses of famciclovir, an anti-viral nucleoside analog, and celecoxib, a COX-2 inhibitor that also possesses unique anti-viral activity. Suppressing latent herpes viruses may significantly improve fibromyalgia-related symptoms. We are currently meeting the development requirements to begin Phase 3 trials with IMC-1 in 2017.”
in order to be granted FDA Fast Track designation, a product must demonstrate potential to address unmet medical need in a serious condition. Companies granted Fast Track designation benefit from greater access to, and more frequent communications with, the FDA throughout the drug development or review process and are allowed to submit New Drug Applications (NDA) on a rolling basis. In addition, Fast Track designation products are eligible for potential accelerated approval and priority review.